Jang Laboratory
 Leveraging epigenetic and metabolic vulnerabilities to improve immunotherapy
Leveraging epigenetic and metabolic vulnerabilities to improve immunotherapy
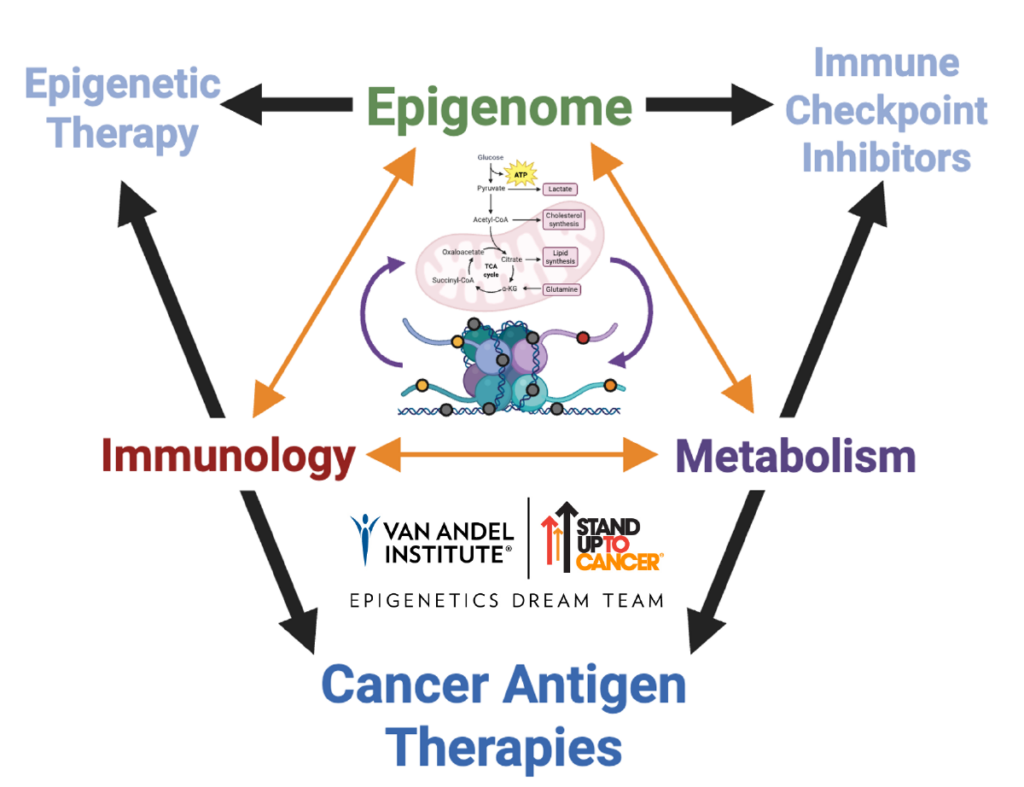
A tumor’s ability to evade immune detection and elimination is a hallmark of cancer. Tumors bypass the immune system by expressing immune checkpoints, which “exhaust” immune cells so that they can no longer recognize and kill cancer cells. Immune checkpoint inhibitors (ICIs) can block these signaling pathways to revitalize immune cell function. However, not every person or every cancer responds to immunotherapies. There is a critical need to understand the mechanisms that contribute to resistance.
The Jang Lab investigates how the crosstalk between metabolism and epigenetics contributes to cancer progression. To this end, we are searching for epigenetic and metabolic vulnerabilities that can be combined with therapeutics to improve cancer immunotherapy treatments.
Epigenetics and metabolism both play roles in cancer. They are tightly linked, with metabolites providing the basic building blocks for epigenetic marks. Unlike changes to DNA, problems with epigenetics and metabolism are reversible, which make them useful targets in cancer treatment. Targeting these vulnerabilities may help the immune system to better spot cancer cells by triggering an internal alarm system.
The Jang Lab also participates in translational research through the Van Andel Institute– Stand Up To Cancer® Epigenetics Dream Team, a multi-institutional collaboration that evaluates promising combination therapies for cancer in clinical trials.
News & Recent Publications
Learn More
Van Andel Institute appoints cancer epigenetics expert Dr. H. Josh Jang as assistant professor
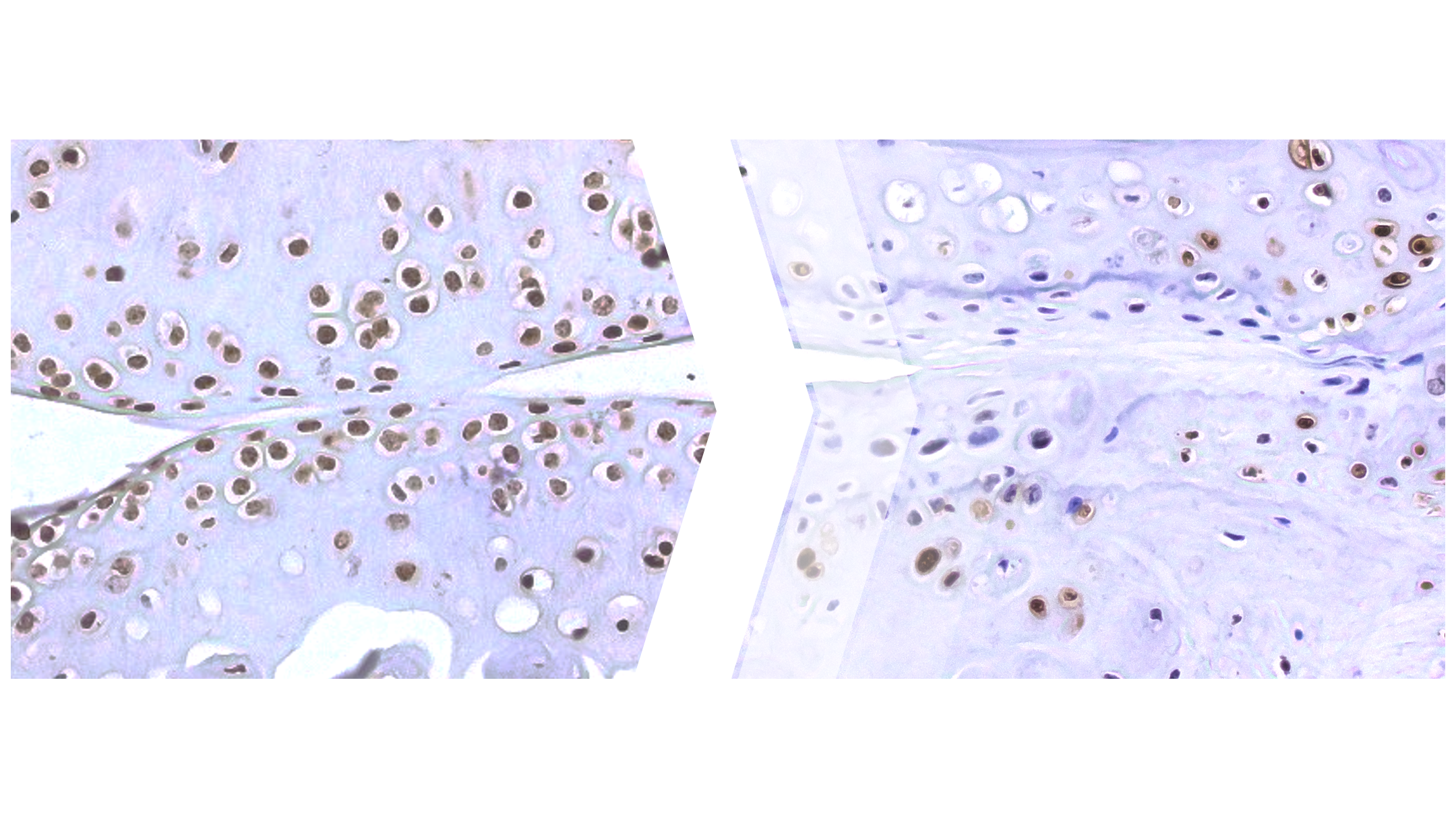
Could silencing old viruses help treat osteoarthritis?

Disrupting Asxl1 gene prevents T-cell exhaustion, improving immunotherapy
Jang HJ*, Shah NM*, Maeng J*, Liang Y*, Basri NL, Ge J, Qu X, Mahlokozera T, Annamalai D, Chen JY, Lee HJ, DeSouza P, Li D, Xing X, Kim AH, Wang T. 2024. Epigenetic therapy potentiates transposable element transcription to create tumor-enriched antigens in glioblastoma cells. Nat Genet 56(9):1903–1913.
Jang HJ, Hostetter G, Macfarlane IV AW, Madaj Z, Ross EA, Hinoue T, Kulchycki JR, Burgos RS, Tafseer M, Alpaugh RK, Schwebel CL, Kokate R, Geynisman DM, Zibelman MR, Ghatalia P, Nichols PW, Chung W, Madzo J, Hahn NM, Quinn DI, Issa JJ, Topper MJ, Baylin SB, Shen H, Campbell KS, Jones PA, Plimack ER. 2023. A phase II trial of guadecitabine plus atezolizumab in metastatic urothelial carcinoma progressing after initial immune checkpoint inhibitor therapy. Clin Cancer Res 29(11):2052–2065.
Li HT*, Jang HJ*, Rohena-Rivera K*, Liu M, Gujar H, Kulchycki J, Zhao S, Billet S, Zhou X, Weisenberger DJ, Gill I, Jones PA, Bhowmick NA, Liang G. 2023. RNA mis-splicing drives viral mimicry response after DNMTi therapy in SETD2-mutant kidney cancer. Cell Rep 42(1):112016.
*Equal contribution
Our Impact
We’re raising thousands to save millions.
We’re turning hope into action for the millions of people around the world affected by diseases like cancer and Parkinson’s. Find out how you can help us make a difference.
- 122 peer-reviewed papers published in 2024, 63 of which were in high-impact journals
- 15 VAI-SU2C Epigenetics Dream Team clinical trials launched to date
- 10 clinical trials co-funded by VAI & Cure Parkinson's (out of 41 total International Linked Clinical Trials Program trials)
H. Josh Jang, Ph.D.
Assistant Professor, Department of Cell Biology
Areas of Expertise
Epigenetic therapy, immunotherapy, cancer, multi-omics, transposable elements, RNA splicing
Biography
Dr. H. Josh Jang investigates combining epigenetic therapies and immune checkpoint inhibitors to enhance the immune system’s ability to better fight cancer.
He earned his B.S. in health promotion and disease prevention from University of Southern California and his Ph.D. in molecular genetics and genomics from Washington University in St. Louis. During his graduate studies in the lab of Dr. Ting Wang, Dr. Jang concentrated on characterizing transposable elements’ contribution to oncogenic potential in cancer cell line models, as well as other projects that established and optimized targeted epigenetic technologies using CRISPR-Cas9 technology.
In 2020, Dr. Jang joined Van Andel Institute as a VAI Fellow under Drs. Peter A. Jones and Stephen B. Baylin. He has received several prestigious awards for his research including a K99/R00 Pathway to Independence grant and a SPORE Epigenetic Therapies: Career Enhancement Program Award, both from the National Cancer Institute.
Dr. Jang also serves as a reviewer for leading journals such as Nature Genetics, Nature Communications, Proceedings of the National Academy of Science and Genome Research, among others. In 2021, he was selected as a Forbeck Scholar and, in 2023, he chaired the Gordon Research Seminar for Cancer Genetics and Epigenetics.
In 2025, Dr. Jang joined Van Andel Institute’s Department of Cell Biology as an assistant professor. He leverages innovative -omics technologies, such as single-cell, spatial and long-read sequencing methods to interrogate how the tumor microenvironment responds to therapy.
Selected Publications
2025
Liu Y, Molchanov V, Zhao Y, Lu D, Liu H, Jang HJ, Yang T. 2025. H3K9me3 and ERV activation as hallmarks for osteoarthritis progression and knee join aging. Osteoarthritis Cartilage 33(1):128–133.
2024
Oswald BM, DeCamp LM, Longo J, Bunda N, Dahabieh M, Ma S, Watson MJ, Sheldon RD, Vincent M, Johnson B, Jones PA, Shen H, Williams K, Crawford PA, Kaech S, Jang HJ, Krawczyk CM, Jones RG. 2024. Pre.print. Dietary restriction enhances CD8 T cell ketolysis to limit exhaustion and boost anti-tumor immunity. bioRxiv
Jang HJ, Urrutia G, Orskov AD, Kim HJ, Nelson SA, Nguyen AV, Lee H, Johnson BK, Mikkelsen SU, Wegener M, Becker K, Adams M, Sheridan R, Ramjan Z, Givan S, Burgos RS, Zebley CC, Youngblood BA, Issa JP, Topper MJ, Baylin SB, Triche Jr. TJ, O’Connell CL, Gronbaek K, Jones PA. 2024. Pre-print. Bone marrow microenvironment signatures associate with patient survival after guadecitabine and atezolizumab therapy in HMA-resistant MDS.
Kang TG, Lan X, Mi T, Chen H, Alli S, Lim SE, Bhatara S, Vasandan AB, Ward G, Bentivegna S, Jang HJ, Spatz ML, Han JH, Schlotmann BC, Jespersen JS, Derenzo C, Vogel P, Yu J, Baylin SB, Jones PA, O’Connell C, Gronbaek K, Youngblood B, Zebley CC. 2024. Epigenetic regulators of clonal hematopoiesis control CD8 T cell stemness during immunotherapy. Science 386(6718):eadl4492.
Jang HJ*, Shah NM*, Maeng J*, Liang Y*, Basri NL, Ge J, Qu X, Mahlokozera T, Annamalai D, Chen JY, Lee HJ, DeSouza P, Li D, Xing X, Kim AH, Wang T. 2024. Epigenetic therapy potentiates transposable element transcription to create tumor-enriched antigens in glioblastoma cells. Nat Genet 56(9):1903–1913.
Ohtani H, Liu M, Liang G, Jang HJ, Jones PA. 2024. Efficient activation of hundreds of LTR12C elements reveals cis-regulatory function determined by distinct epigenetic mechanisms. Nucleic Acids Res 52(14): 8205–8217.
2023
Maeng JH, Jang HJ, Du AY, Tzeng SC, Wang T. 2023. Using long-read CAGE sequencing to profile cryptic- promoter-derived transcripts and their contribution to the immunopeptidome. Genome Res 33(12):2143–2155.
Shah NM*, Jang HJ*, Liang Y*, Maeng JH, Tzeng SC, Wu A, Basri NL, Qu X, Fan C, Li A, Katz B, Li D, Xing X, Evans BS, Wang T. 2023. Pan-cancer analysis identifies tumor-specific antigens derived from transposable elements. Nat Genet 55(4):631–639.
Jang HJ, Hostetter G, Macfarlane IV AW, Madaj Z, Ross EA, Hinoue T, Kulchycki JR, Burgos RS, Tafseer M, Alpaugh RK, Schwebel CL, Kokate R, Geynisman DM, Zibelman MR, Ghatalia P, Nichols PW, Chung W, Madzo J, Hahn NM, Quinn DI, Issa JJ, Topper MJ, Baylin SB, Shen H, Campbell KS, Jones PA, Plimack ER. 2023. A phase II trial of guadecitabine plus atezolizumab in metastatic urothelial carcinoma progressing after initial immune checkpoint inhibitor therapy. Clin Cancer Res 29(11):2052–2065.
Li HT*, Jang HJ*, Rohena-Rivera K*, Liu M, Gujar H, Kulchycki J, Zhao S, Billet S, Zhou X, Weisenberger DJ, Gill I, Jones PA, Bhowmick NA, Liang G. 2023. RNA mis-splicing drives viral mimicry response after DNMTi therapy in SETD2-mutant kidney cancer. Cell Rep 42(1):112016.
Xing X, Karlow JA, Li D, Jang HS, Lee HJ, Wang, T. 2023. Capture methylation-sensitive restriction enzyme sequencing (Capture MRE-Seq) for methylation analysis of highly degraded DNA samples. Methods Mol Biol 2621:73–89.
2022
O’Connell CL, Baer MR, Orskov AD, Saini SK, Duong VH, Kropf P, Hansen JW, Tsao-Wei D, Jang HS, Emadi A, Holmberg-Thyden S, Cowland J, Brinker BT, Horwood K, Burgos R, Hostetter G, Youngblood BA, Hadrup SR, Issa JP, Jones PA, Baylin SB, Siddiqi I, Gronbaek K. 2022. Safety, outcomes, and T-cell characteristics in patients with relapsed or refractory MDS or CMML treated with atezolizumab in combination with guadecitabine. Clin Cancer Res 28(24): 5306–5316.
Karlow JA, Devarakonda S, Xing X, Jang HS, Govindan R, Watson M, Wang T. 2022. Developmental pathways are epigenetically reprogrammed during lung cancer brain metastasis. Cancer Res 82(15):2692–2703.
2021
Jang HS*, Chen Y*, Ge J, Wilkening AN, Hou Y, Lee HJ, Choi YR, Lowden RF, Xing X, Li D, Kaufman CK, Johnson SL, Wang T. 2021. Epigenetic dynamics shaping melanophore and iridophore cell fate in zebrafish. Genome Biol 22(1):282.
*Equal contribution


Thomas Goralski, Ph.D., M.S.
Postdoctoral Fellow, Jang Laboratory

Ben Johnson, Ph.D.
Senior Research Scientist, Department of Epigenetics & Department of Cell Biology
Epigenetics of reproductive cancers

Hyein Lee
Assistant Research Technician, Department of Cell Biology

Amy Nuffesse
Senior Administrative Assistant II, Department of Epigenetics

Steve Pierce, Ph.D.
Computational Biologist III, Department of Cell Biology

Young Sung Lee
Assistant Research Technician, Department of Cell Biology

